ZnO nanoparticles as photodegradation agent controlled by morphology and boron doping

Daniel Furka, Samuel Furka, Mira Naftaly, Erik Rakovský, Mária Čaplovičová, Marián Janek
Catal. Sci. Technol., 2021,11, 2167-2185
https://doi.org/10.1039/D0CY01802C
Abstract
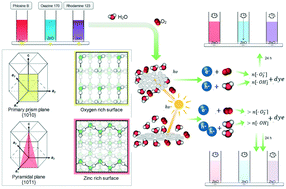
Photolytic degradation of model dyes on pure and boron-doped zinc oxide (ZnO) nanoparticles with pseudohexagonal or elongated spindle-like morphology was investigated. ZnO nanoparticles were prepared by spray-assisted co-precipitation solvothermal synthesis. The bandgap (Eg) of pure ZnO was decreased by boron doping. The prepared nanoparticles were tested for their photocatalytic activity by decomposition of different organic dyes, using phloxine, oxazine and rhodamine as test substances simulating environmental pollutants. The photolysis experiments were done in a 3D printed photoreactor using a photodiode with a wavelength of 365 ± 5 nm as a narrow-line radiation source. The observed first-order reaction kinetics revealed that higher reaction rates were achieved on pseudohexagonal nanoparticles. Increasing the boron content led to accelerated photolysis rates. A significant linear correlation was observed between the optical bandgap energy Eg and the residual dye concentration remaining after 6 hours from the start of the reaction. It was found that ZnO particles with pseudohexagonal morphology decompose organic dyes faster than elongated spindle-like particles, indicating dependence on the surface area as determined by BET analysis. Interestingly, the residual dye concentration varied more strongly with doping when elongated spindle-like nanoparticles were used. It was shown that the efficiency of a photolysis reaction occurring on a solid oxide/solution interface is affected by the crystallographic plane on which it takes place.
